WHAT IS ZEOLITE?
Frederick Mumpton calls it La Roca Magica, “The Magic Mineral”, at his most cited article, ” The uses of natural zeolites in agriculture and industry”. Mumpton calls it magical due to its multitude of uses like in pozzolanic cement; as lightweight aggregates; in the drying of acid-gases; in the separation of oxygen from air; in the removal of NH3 from drinking water and municipal wastewater; in the extraction of Cs and Sr from nuclear wastes and the mitigation of radioactive fallout; as dietary supplements to improve animal production; as soil amendments to improve cation-exchange capacities (CEC) and water sorption capacities; as soilless zeoponic substrate for greenhouses and space missions; in the deodorization of animal litter, barns, ash trays, refrigerators, and athletic footwear; in the removal of ammoniacal nitrogen from saline hemodialysis solutions; and as bactericides, insecticides, and antacids for people and animals. We are proud to announce that the usage areas of zeolite have already been doubled during the last 20 years. Now you can find zeolite in your toothpastes, filters, clothes, in your cosmetics, maybe at your soap, in your wall or window, inside your refrigerator, air conditioner. Surely inside several things at your home that you would never guess.
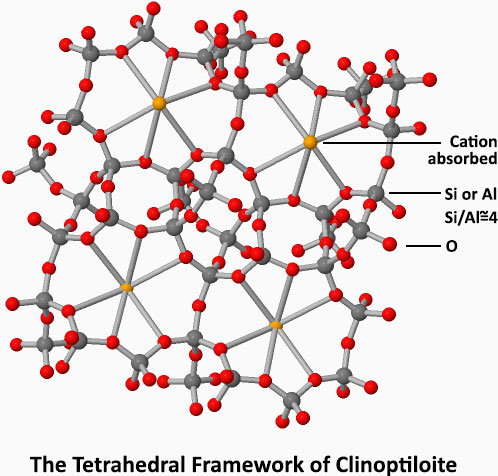
Mumpton describes the zeolite as a “crystalline, hydrated aluminosilicate of alkali and alkaline earth cations having an infinite, open, threedimensional structure. It is further able to lose and gain water reversibly and to exchange extraframework cations, both without change of crystal structure. The large structural cavities and the entry channels leading into them contain water molecules, which form hydration spheres around exchangeable cations.”
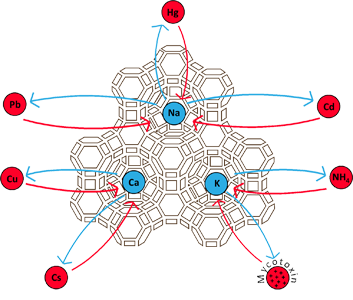
Zeolite is a magnet that can hold cations, like heavy metal, ammonia, low level radioactive elements, toxins, several odours, petrochemicals, many different type of gases and a multitude of various solutions. It is a highly porous sponge with a large surface area that can absorb water up to 40% of its weight.
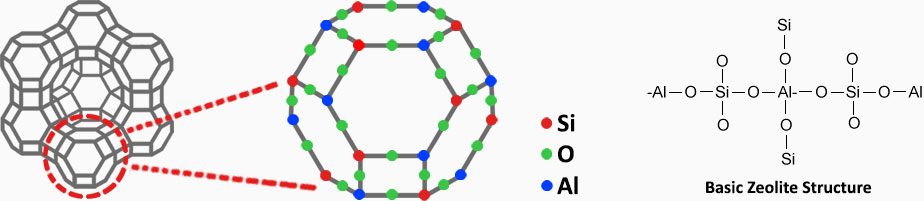
The magic of the mineral lies at its cage structure and at its Aluminium and Silicon content which enables the mineral a high cation exchange capacity.
WHAT IS CLINOPTILOLITE?
There are more than 50 types of natural zeolite mineral. Clinoptilolite (from the heulandite group) is the most common and suitable natural zeolite type for commercial and industrial applications. More than 90% of the world’s zeolite production is clinoptilolite type of zeolite. Apart from natural ones, many synthetic zeolite are being developed around the world for
custom applications.
The descriptive image of zeolite at the left is taken from ChemTube3D. Please visit their website for an informing 3d animation of clinoptilolite’s tetrahedral structure and how it absorbs the cations into its cage like a magnet.
Currently most of the natural zeolite products at the market are clinoptilolite. However, not all zeolite products have a high content of clinoptilolite. The quality of a zeolite mineral totally depends on the clinoptilolite content of the product since the active agent (that can exchange cations) in the mineral is the clinoptilolite. Unfortunately at the market there are products that have clinoptilolite content as low as 50%, even sometimes 40%, which means that rest of the product is regular stone or clay.
Low quality products usually originate in China and are mostly used as aggregate. This aggregate quality clinoptilolite mineral shall not be compared with the high quality (min. 80% content) clinoptilolite minerals.
The clinoptilolite series comprises three species. Clinoptilolite-K, Clinoptilolite-Na, Clinoptilolite-Ca named after their dominant elements. These elements are exchanged during cation exchange in favor of heavy metals, toxins, ammonia etc. which have a higher attraction for the mineral. To check how these elements are placed inside the clinoptilolite structure please check The Virtual Museum of Minerals and Molecules.
 MARUTI ZEOLITE
MARUTI ZEOLITE